Bulletin #1087, Soil pH
By Steven B. Johnson, Ph.D., Extension Crops Specialist, and John Jemison, Extension Professor, Soil and Water Quality, University of Maine Cooperative Extension
For information about UMaine Extension programs and resources, visit extension.umaine.edu.
Find more of our publications and books at extension.umaine.edu/publications/.

Soil pH is the measurement of “potential of hydrogen.” It is a measure of the relative acidity of soil, which is the amount of hydrogen in the soil solution. Soil pH is the negative logarithm of the activity of hydrogen ions (H+) in the soil solution (a soil pH of six has 0.000001 eq/L of H). It is not a measure of the total acidity in the soil. To determine total acidity of a soil, one must also determine the percentage of the soil exchange sites occupied by H+ and Al3+.
The pH identifies a soil as acidic, neutral, or alkaline. pH is measured on a scale of 1 to 14, where pH 7 is neutral, pH less than 7 is acidic, and pH greater than 7 is alkaline. The base 10 logarithmic pH scale has a ten-fold increase in acidity for every unit change in pH. For instance, a soil with pH 6 has 10 times the acidity of a pH 7 soil. Likewise, a pH 5 soil has 100 times the acidity of a pH 7 soil. For reference, the human stomach has a pH from 1.5 to 3.5, vinegar about 2.4, cola about 2.5, blood about 7.4, and household bleach about 12.
For many soils in Maine that have not been cropped or amended with lime, the natural soil pH is between 4.5 and 5, and the soil exchange is dominated by H+ and Al3+. If limed and fertilized, the cations (positively charged ions) calcium (Ca+2), magnesium (Mg+2), and potassium (K+) will dominate the exchange sites with the increased soil pH. H+ and Al3+ held on exchange sites would be lowered. Growers should aim to have less than 5% of their soil exchange sites occupied by H+ and Al3+.
The amount of lime required to neutralize the active acidity in the soil solution (H+ and Al3+) is quite low relative to the amount of lime required to neutralize the reserve acidity on the soil sites occupied by H+ and Al3+. With that, the amount and balance of Ca+2, Mg+2, and K+ (also constituents of the soil exchange) indirectly influence the amount of reserve acidity and as such, the relative health of the soil.
Application of Ca+2, Mg+2, K+, and others (as fertilizers or liming agents) increase their amount on the soil exchange by replacing H+ and Al3+ on soil particles. Water flowing through the soil (which can leach Ca+2, Mg+2, and K+), plant growth, decomposition of organic matter, oxidative weathering of soils will allow H+ and Al3+ to return to soil sites. This, and particularly, application of nitrogen fertilizers, increase both the active and reserve acidity soil acidity and contribute to lowering soil pH.
A proper balance of these cations in the soil encourages microbial activity and enhances soil structure and nutrients available for plant growth.
Under acidic conditions, H+ dominates the exchange sites on soil particles. When this occurs, it limits the exchange of other cations needed for plant growth. While some elements may be present in the soil at adequate levels, they are not available for plant growth.
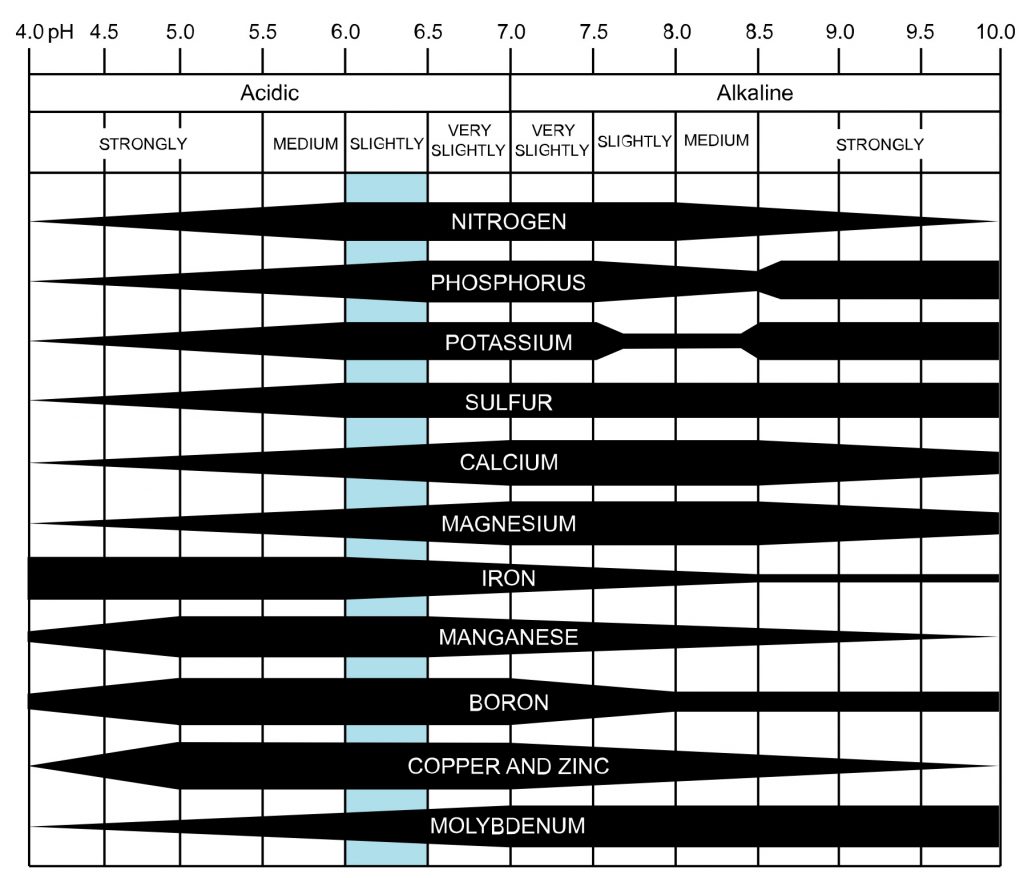
Figure 1 shows the availability of nutrients at a range of soil pH values.
In very acidic soils, nitrogen, phosphorus, potassium, sulfur, calcium, manganese, and molybdenum have reduced availability to the point that they may be unavailable. Under very low pH conditions, even the addition of fertilizer may not overcome nutrient deficiency symptoms. Acidic soils have iron, aluminum, and manganese very available to the point of toxicity.
A very acidic soil may have good water drainage and good physical characteristics, but aluminum may limit plant root growth. Succumbing to drought is a common result of poor and damaged root growth resulting from excessive aluminum. Manganese toxicity is also a problem with very acidic soils, as are deficiencies of magnesium and calcium.
Aluminum is present in all soils but is toxic to most plants when in solution. At higher soil pH, aluminum is found in a form called gibbsite (AlOH3) which is unavailable to plants. The solubility of aluminum increases as the soil pH decreases. Al3+ toxicity is a major constraint to crop production. Al is not an essential plant nutrient. When in solution, it can enter plant roots via osmosis (spontaneous flow through a membrane). Root tips may turn brown, and normal root processes of growth and nutrient absorption are interfered with.
Soil pH dictates nutrient reactions in the soil solution. Under acidic conditions, the pH in solution is buffered by aluminum, rather than by calcium, potassium, or magnesium. Under these conditions, aluminum hydroxides are formed. Raising the soil pH above 5.5 causes most of the aluminum complexes to precipitate, or change into forms unavailable to plants.
Alkaline soils tend to have excess calcium, which also limits availability of phosphorus, copper, manganese, zinc, iron, and boron, which are essential nutrients for plants. Molybdenum may become more available in alkaline soils.
Reviewed by Ellen Mallory, Extension professor, Sustainable Agriculture, University of Maine Cooperative Extension
Information in this publication is provided purely for educational purposes. No responsibility is assumed for any problems associated with the use of products or services mentioned. No endorsement of products or companies is intended, nor is criticism of unnamed products or companies implied.
© 2020
Call 800.287.0274 (in Maine), or 207.581.3188, for information on publications and program offerings from University of Maine Cooperative Extension, or visit extension.umaine.edu.
In complying with the letter and spirit of applicable laws and pursuing its own goals of diversity, the University of Maine System does not discriminate on the grounds of race, color, religion, sex, sexual orientation, transgender status, gender, gender identity or expression, ethnicity, national origin, citizenship status, familial status, ancestry, age, disability physical or mental, genetic information, or veterans or military status in employment, education, and all other programs and activities. The University provides reasonable accommodations to qualified individuals with disabilities upon request. The following person has been designated to handle inquiries regarding non-discrimination policies: Director of Institutional Equity and Title IX Services, 5713 Chadbourne Hall, Room 412, University of Maine, Orono, ME 04469-5713, 207.581.1226, TTY 711 (Maine Relay System).