Bulletin #2249, Nitrogen: An Essential Element for Potatoes
Bulletin #2249, Nitrogen: An Essential Element for Potatoes (PDF)
By Steven B. Johnson, Ph.D., Extension Crops Specialist, University of Maine Cooperative Extension
Reviewed by John Jemison, ExtensionProfessor, Soil and Water Quality, University of Maine Cooperative Extension
For information about UMaine Extension programs and resources, visit extension.umaine.edu.
Find more of our publications and books at extension.umaine.edu/publications/.
Nitrogen (N) is a macronutrient essential for plant growth. Potassium (K) and phosphorus (P) are also essential macronutrients. Fertilizer that is listed as 15-15-15 contains N-P2O5-K2O in the ratio of 15-15-15 on a percentage basis and this blend contains 15 percent elemental nitrogen, or N.
Potato varieties vary, but a crop of 300 hundredweight of potato tubers and associated vines needs around 200 pounds of N per acre. The University of Maine soil test reports give P and K test levels and recommendations, but a basic soil test provides only an N recommendation owing to the changing status of N in soils.
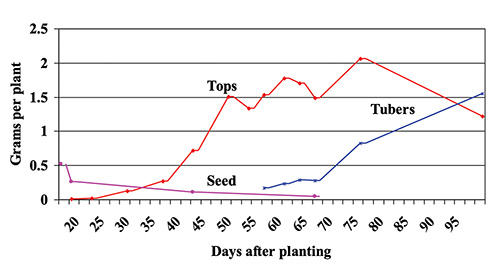 The Role of N in Plants
The Role of N in Plants
N has an integral role in plant protein. It is also an essential component of amino acids, which form proteins. N increases the protein content of plants. N is needed for chlorophyll synthesis, a component in plant energy systems.
N Deficiency in Plants
N deficiency is characterized by yellowing of the leaves. This is a result of inadequate chlorophyll. The yellowing symptoms start on the oldest leaves and develop on younger leaves as the deficiency continues. This is quickly reversed with the application of N. N-deficient plants will have reduced growth, delayed maturity, and reduced yields when compared to adequately fertilized plants.
Plant-Available N
Unavailable N
The unavailable portion of the N in soils is called organic N. This constitutes over 95% of the total N in soils. Organic N is not immediately available for plant growth through the mineralization process mentioned below.
Available N
The plant-available portion of the N in soils is called inorganic N. Plant-available N forms include ammonium (NH4+) and nitrate (NO3–).
N Fixation
Symbiotic N fixation occurs with Rhizobium bacteria. These bacteria are associated with the roots of legumes such as alfalfa, clover, and soybean. These bacteria convert atmospheric N gas (N2) to ammonium (NH4+).
N Transformations
Mineralization and Immobilization
Mineralization is the process through which microbial activity breaks down organic N, such as from decaying plant material, making some of it available for plant growth.
Immobilization is the process of inorganic N in soil being converted to organic N, making it unavailable for plant growth. Effectively, the N is temporarily tied up by microorganisms in the soil. This effectively moves the N from available to unavailable status in the soil. Immobilization occurs when high carbon-to-nitrogen ratio crop residue, such as straw or sawdust, is incorporated into the soil.
The rates of mineralization and denitrification are affected by typical factors influencing biological activity, such as temperature, moisture, oxygen, and pH. If immobilization exceeds mineralization, there may be almost no N available for plant growth from the soil. If this occurs, there is a N depression period. Avoiding this is a main reason to have some starter fertilizer available near the plant roots. Incorporating plant residues well in advance to allow adequate decomposition will reduce the N depression period.
Ammonification
An early breakdown product during mineralization is ammonium (NH4+). This process is called ammonification. NH4+ is readily absorbed on cation exchange capacity (CEC) sites on clay and organic matter and is stable in the soil. It is also not subject to denitrification.
Nitrification
Nitrification is a two-step process accomplished by nitrifying soil bacteria that converts ammonium to nitrate (NO3–). In the first step, nitrite (NO2–) is formed from NH4+. This step is carried out by soil bacteria frequently in the Nitrosomonas genus. This process requires oxygen and decreases the soil pH by the release of hydrogen ions (H+). The second step is the transformation of NO2– to NO3–. Soil bacteria, frequently in the genus Nitrobacter, perform this last step. Nitrate is released in the soil, but being very mobile, does not accumulate to any great degree in soil.
N Loss Mechanisms
Denitrification
Denitrification is one way N is lost from soils. Under anaerobic conditions, which occur with waterlogged soils, some microorganisms use NO3– after the oxygen (O2) is depleted. Nitrous oxide (N2O) and N gas (N2) are released as a result of the anaerobic microorganism activity.
Volatilization
Volatilization is another means of N loss from soils. This occurs from the conversion of NH4+ to ammonia gas (NH3–). This also decreases the soil pH by the release of H+. This loss can be as much as 50% if urea fertilizers are not incorporated or watered in. Losses can occur in the range of 20% in a week.
Leaching
Leaching is a major loss mechanism for soil N. Much of the applied fertilizer has the potential to leach when in the NO3– form. NO3– is highly soluble and readily leached and has contaminated subsurface water systems. Cropping removes N from the soil, but this is not leaching.
N Reaction
The conversion of NH4+ to NO3– releases H+ into the soil solution. This increases soil acidity. The NO3– ion is a factor associated with leaching of basic cations from the soil. As NO3– ions and calcium (Ca+2), magnesium (Mg+2), and potassium (K+) move out together, the cations are replaced with H+, leading to increased acidity.
| Source | Analysis (N-P-K) |
|---|---|
| Ammonium sulfate (NH4SO4) | 21-0-0 |
| Ammonium nitrate (NH4NO3) | 34-0-0 |
| Urea (CO(NH2)2) | 46-0-0 |
| Urea ammonium nitrate (CO(NH2)2+ NH4NO3) | 28-0-0 to 32-0-0 |
| Calcium nitrate (Ca(NO3) 2) | 15.5-0-0 |
| Calcium ammonium nitrate | 25-0-0 |
Information in this publication is provided purely for educational purposes. No responsibility is assumed for any problems associated with the use of products or services mentioned. No endorsement of products or companies is intended, nor is criticism of unnamed products or companies implied.
© 2020
Call 800.287.0274 (in Maine), or 207.581.3188, for information on publications and program offerings from University of Maine Cooperative Extension, or visit extension.umaine.edu.
In complying with the letter and spirit of applicable laws and pursuing its own goals of diversity, the University of Maine System does not discriminate on the grounds of race, color, religion, sex, sexual orientation, transgender status, gender, gender identity or expression, ethnicity, national origin, citizenship status, familial status, ancestry, age, disability physical or mental, genetic information, or veterans or military status in employment, education, and all other programs and activities. The University provides reasonable accommodations to qualified individuals with disabilities upon request. The following person has been designated to handle inquiries regarding non-discrimination policies: Director of Institutional Equity and Title IX Services, 5713 Chadbourne Hall, Room 412, University of Maine, Orono, ME 04469-5713, 207.581.1226, TTY 711 (Maine Relay System).

