Bulletin #2250, Phosphorus: An Essential Element for Potatoes
Bulletin #2250, Phosphorus: An Essential Element for Potatoes (PDF)
By Steven B. Johnson, Ph.D., Extension Crops Specialist, University of Maine Cooperative Extension
Reviewed by John Jemison, ExtensionProfessor, Soil and Water Quality, University of Maine Cooperative Extension
For information about UMaine Extension programs and resources, visit extension.umaine.edu.
Find more of our publications and books at extension.umaine.edu/publications/.
Phosphorus (P) is a macronutrient essential for plant growth. Nitrogen (N) and potassium (K) are also essential macronutrients. Fertilizer that is triple 15 contains N-P-K in the ratio of 15-15-15 on a percentage basis. However, the P is not 15% P; it is actually 15% P2O5. Fifteen percent P2O5 translates to 6.5% elemental P.
A crop of 300 hundredweight of potato tubers and associated vines needs 12–20 pounds of elemental P, or about 30–45 pounds of P2O5 per acre. P use by the potato plant is about one-sixth the amount of N used. The University of Maine soil test reports give P recommendations
The Role of P in Plants
P plays a key role in energy transfer in plants. Energy storage and release through ATP (adenosine triphosphate) molecules require P. Photosynthesis and respiration, as well as nucleic material, such as DNA, require P. Cell division and expansion and other energy-intensive processes during growth require high concentrations of P. In the plant, P is mobile, moving from older tissue to newer tissue. The highest levels of P are found in tissue at the growing point.
P is key in helping roots develop more rapidly and increase water use efficiency. Seeds contain a higher proportion of P than do other parts of the plant. P deficiency is most common in older parts of the plant. Lower leaves on potato plants show P deficiency symptoms before newer, upper leaves.
Most P is absorbed by plants in the form of the primary orthophosphate ion (H2PO4–1) and, to a lesser extent, as the secondary orthophosphate ion (HPO4–2). In some cases, other forms of P are taken up, but to a much lesser extent than either of these two orthophosphate ions.
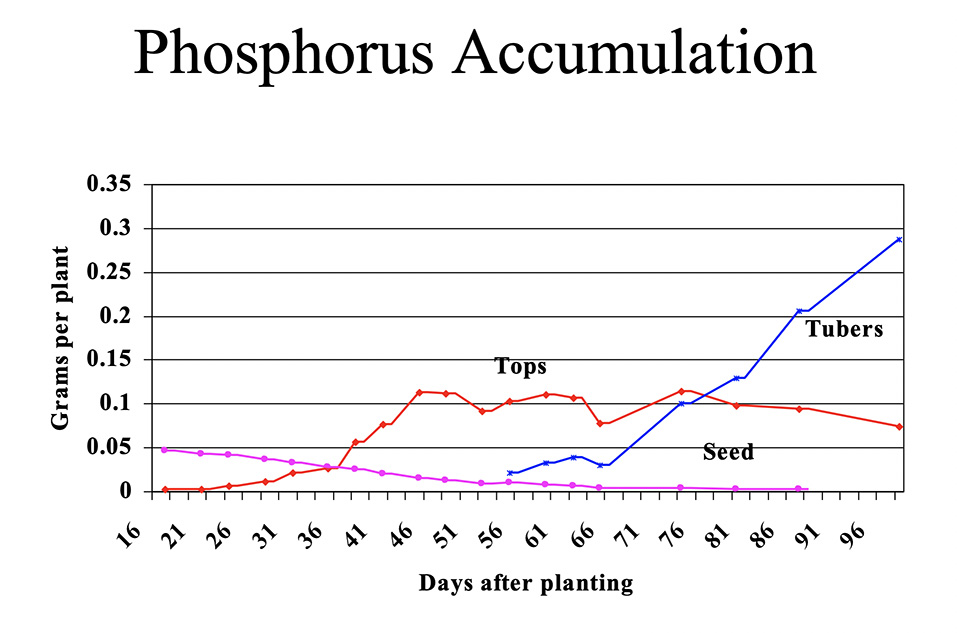
Potatoes accumulate P in the tops and tubers and lose P from the seed piece. Figure 1 shows P accumulation in potatoes grown in Aroostook County, Maine.
P Deficiency in Plants
Potato plants deficient in P will be smaller than normal, somewhat spindlier in appearance, and generally behind in development, especially early in the season. Overall, potato plants deficient in P appear as stunted plants. P-deficient foliage is frequently darker green than normal foliage. Severe P deficiency results in an upward cupping of the leaf blades, revealing the gray-green color on the lower surface. Tubers are reported to have rusty brown isolated flecks. Most Maine soils have naturally moderately high levels of soil P, and with high fertilization rates over many years, visible P deficiency symptoms in Maine potatoes are rare. But if P is limited, it is reflected more in low yields and poor-quality tubers than in distinctive foliar symptoms.
P Movement in Soils
Since elemental P is very reactive chemically, it is not found as elemental P in nature, but as a compound. Soil P comes mainly from the weathering of the mineral apatite. As apatite breaks down, primary orthophosphate ion (H2PO4–1) and secondary orthophosphate ion (HPO4–2) are formed. These ions are released into the soil solution and are absorbed by plant roots. Soluble P will form compounds with calcium (Ca) at high pH, and iron (Fe) and aluminum (Al) at lower pH. Most of these compounds formed are fixed and are not available to the plant. In fact, some soils may have a half ton of P per acre, almost all of it unavailable to plants. There are usually only about 4 pounds per acre of plant-available P in the soil solution at any one time in Maine soils. Estimates of the crop being able to take up less than 20% of the applied P are generous under some soil pH conditions. The key to proper P fertilization is not the level of P in the soil, but the ability of the soil to replace the P the plant removes from the soil solution. This replacement may occur several times per day or hundreds of times during the growing season.
P, under most circumstances, has limited mobility in soils; little is lost to leaching. The majority of the P stays where it is put. P moves by diffusion and is very dependent on soil moisture. It is very unlikely that P placed 0.5 millimeters away from a potato root will move close enough to be taken up by the root. P needs to be placed where the roots can intercept it. Soil erosion can move P and has been a problem in some watersheds. Crop removal is the other major method of P removal from soils.
Clay will fix P, so soils high in clay rapidly convert P to a form unavailable to plants. Anything that reduces aeration of soils, whether compaction or excess water, reduces P in soils. Microbial breakdown of organic matter contributes greatly to P in the soil solution, and reducing aeration reduces microbial activity. N can have an effect on P uptake by plants. Ammonium (NH4+) in high concentrations slows P fixation reactions. Ammonium absorption helps maintain an acidic soil condition at the root surface and improve P absorption by the soil.
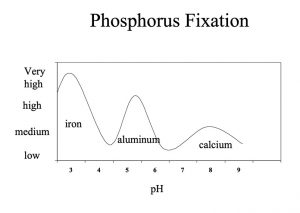
The largest effect on soil P is soil pH. In acidic soils (soils below pH 7), P reacts with Fe, manganese (Mn), and Al, among other elements. These reactions fix P, making it unavailable to the plant. Figure 2 shows the influence of soil pH level on P fixation and the primary elements involved.
A low pH in some soils allows Al to react with P and make it unavailable for plant uptake. Banded placement of P in some cold soils has proven to be beneficial. Lower levels of P are needed when it is banded rather than broadcast. The high concentrations obtained with banding just below the potato seed piece locate the P where it will be available to the young, restricted, yet rapidly developing root system.
P Sources
Commercial sources of P are phosphate rock. These are mined and upgraded by removing clay and other impurities. The rock is then finely ground and acid-treated. A small portion of P, mainly the liquid form, is thermally processed. The addition of P to the soil does not lower the soil pH. P tends to stimulate N fixation where nitric acid is the conversion product, which can reduce soil pH.
| Source | Analysis (N-P-K) |
|---|---|
| Superphosphate | 20 |
| Double superphosphate | 43 |
| Triple superphosphate | 46 |
| Monoammonium phosphate (MAP) | 48 |
| Diammonium phosphate (DAP) | 46 |
| Manure | 1 |
Information in this publication is provided purely for educational purposes. No responsibility is assumed for any problems associated with the use of products or services mentioned. No endorsement of products or companies is intended, nor is criticism of unnamed products or companies implied.
© 2020
Call 800.287.0274 (in Maine), or 207.581.3188, for information on publications and program offerings from University of Maine Cooperative Extension, or visit extension.umaine.edu.
In complying with the letter and spirit of applicable laws and pursuing its own goals of diversity, the University of Maine System does not discriminate on the grounds of race, color, religion, sex, sexual orientation, transgender status, gender, gender identity or expression, ethnicity, national origin, citizenship status, familial status, ancestry, age, disability physical or mental, genetic information, or veterans or military status in employment, education, and all other programs and activities. The University provides reasonable accommodations to qualified individuals with disabilities upon request. The following person has been designated to handle inquiries regarding non-discrimination policies: Director of Equal Opportunity and Title IX Services, 5713 Chadbourne Hall, Room 412, University of Maine, Orono, ME 04469-5713, 207.581.1226, TTY 711 (Maine Relay System).

