Bulletin #4280, FSMA Preventive Controls for Human Food Rule Exemptions
Developed by Robson Machado, Assistant Extension Professor and Food Science Specialist, University of Maine Cooperative Extension.
Reviewed by Luke LaBorde, Professor of Food Science, Penn State, Amanda Kinchla, Extension Assistant Professor, Department of Food Science, University of Massachusetts Amhers, and Jason Bolton, Associate Extension Professor and Food Safety Specialist, University of Maine Cooperative Extension.
For information about UMaine Extension programs and resources, visit extension.umaine.edu.
Find more of our publications and books at extension.umaine.edu/publications/.
The Food and Drug Administration (FDA) has created new regulations that will dramatically affect the food industry. The purpose of the Food Safety Modernization Act (FSMA) is to minimize the risk of foodborne diseases in our food system.
The FSMA is one of the largest changes to food safety regulations and will impact how we think about food safety. The FSMA brings forth a more proactive approach, rather than reacting to outbreaks, such as providing food safety risks assessments and identifying steps to control or minimize such risks before a foodborne risk incidence can occur. All food processors required to register with the FDA will be required to comply with the updated Current Good Manufacturing Practices (CGMPs). Some processors may be exempt from the extra requirements that came with the Food Safety Modernization Act (FSMA) depending on their size.
While there are seven rules within FSMA, two of the rules, Produce Safety Rule (PSR) and Preventive Controls for Human Food (PC), have a significant impact on producers and processors within Maine, New England, and the USA. The seven rules are:
- Preventive Controls for Human Food (PC): updated CGMPs for the entire industry and the implementation of a HACCP-like system to sectors not covered under specific HACCP rules.
- Produce Safety (PSR): establishment of practices related to food safety, sanitation, personal hygiene, water safety, soil amendments use, wild and domestic animal handling for produce farms.
- Preventive Controls for Animal Food: similar to the rule for human food that we will discuss in this fact sheet but related to animal food.
- Foreign Supplier Verification Programs: new rules for anyone who imports food into the United States to ensure the same food safety standards required of domestic processors.
- Accredited Third-Party Certification: establishment of a program to accredit third-party auditors to conduct food safety audits and issue certifications for foreign facilities and their food.
- Sanitary Transportation: establishment of sanitary practices to reduce the risk of food becoming contaminated during transportation.
- Intentional Adulteration (Food Defense): more specific for large companies whose food reach many people, this rule aims to protect the food supply against intentional contamination.
The Preventive Controls for Human Food Rule (PC Rule)
Anyone who manufactures, processes, packs, and/or holds any food for human consumption will be expected to comply with at least some parts of these new regulations. Foods and products already covered by other specific regulations (e.g., juice, seafood, low acid canned foods, dietary supplements, and alcohol) must register with the FDA and comply with CGMPs, but are exempt from most of the PC requirements, as long as they comply with their industry-specific regulations.
Companies that fall under the PC rule will have to follow the updated CGMPs (these practices were updated within the PC rule from an older version with the same name). They will also have to write a food safety plan that includes a hazard analysis (including hazard identification and hazard evaluation); establishment of preventive controls for the identified hazards; and oversight and management of the preventive controls through monitoring, corrective actions, validations, and verifications.
Food processors that must fully comply with the PC rule will require someone who went through the Preventive Controls Rule training to become a PCQI (Preventive Controls Qualified Individual) to help them put a food safety plan together. For some companies, the required food safety plan will be a novelty. Facilities that are familiar with Hazard Analysis and Critical Control Point (HACCP) plans will recognize various aspects of HACCP when developing their food safety plan. One of the most important similarities is conducting a comprehensive hazard analysis of the entire food production process. In this analysis, the food processing steps most likely to pose a food safety hazard for consumers must be identified and addressed. After that, preventive controls — hence the name — are established to minimize such hazards. These preventive controls are closely monitored and recorded by workers who are trained to perform the activity and know what to do if something does not work as expected and corrective actions must be taken. Some examples are monitoring and recording heating temperatures for cooked products and checking labels to assure that all potential allergens are listed. Traceability is also an important factor of the new regulation, especially regarding ingredient suppliers. With the integrated food production industry that exists today in the U.S., food may contain ingredients from dozens of suppliers. With the new regulation, companies will have to make sure suppliers are providing safe ingredients by asking for lab test results, performing audits, etc.
Exemptions to the PC Rule
There are some exemptions to the rule depending on the type of activity, type of facility, or company size.
Low-risk on-farm activities
Companies that only perform certain low-risk, on-farm processing and manufacturing activities (e.g., cutting lemons and limes, slicing bread, baking bread, etc.) may be eligible for an exemption to some parts of the rule depending on their size. FDA is very specific about what is considered a low-risk activity and compiled a list of exempt low-risk activities.
Storage facilities
If you only hold (store) packaged food that is not exposed to the environment, you are exempt from most requirements of the rule. However, if the packaged food you hold requires refrigeration, you must be able to prove that you properly controlled the temperature of such foods.
Exemptions based on company size
Even if you are not exempt as outlined above, you may still be eligible for an exemption to some parts of the rule and only have to comply with the Current Good Manufacturing Practices (CGMPs). A facility with that partial exemption is called a “qualified facility.”
The FDA defines a “qualified facility” in the rule as follows:
A “qualified facility” is:
1) A “very small business,” with average annual food sales (plus the market value of food manufactured, processed, packed, or held) of $1,000,000, adjusted for inflation based on 2011 dollars, during the last 3-years;
OR
2) A facility to which both of the following conditions apply during the last 3 years:
(i) the average annual value of the food manufactured, processed, packed or held that is sold directly to qualified end-users exceeded the average annual value of the food sold to all other purchasers;
AND
(ii) the average annual value of all food sold during the previous 3-year was less than $500,000, adjusted for inflation.
A Qualified end-user is:
1) the actual consumer of the food
OR
2) a restaurant or retail food establishment that is purchasing the food for future sales directly to consumers as long as that restaurant or retail food establishment is located:
a. In the same state or the Indian reservation as the qualified facility that sold the food;
OR
b. Not more than 275 miles from the facility.
If you think that your business can claim the status of a qualified facility, you will still have to register your facility with the FDA and submit documentation to the FDA that includes the sales records from the previous three years. Keep in mind that even if you are a qualified exempt facility, you still must conduct a hazard analysis and have preventive controls in place to address any hazards you may have found at your facility. You will also have to comply with any State and local food safety laws that are specific to your location and product. You can find additional information about what to do, if you are a qualified facility, in this FDA qualified facility guidance.
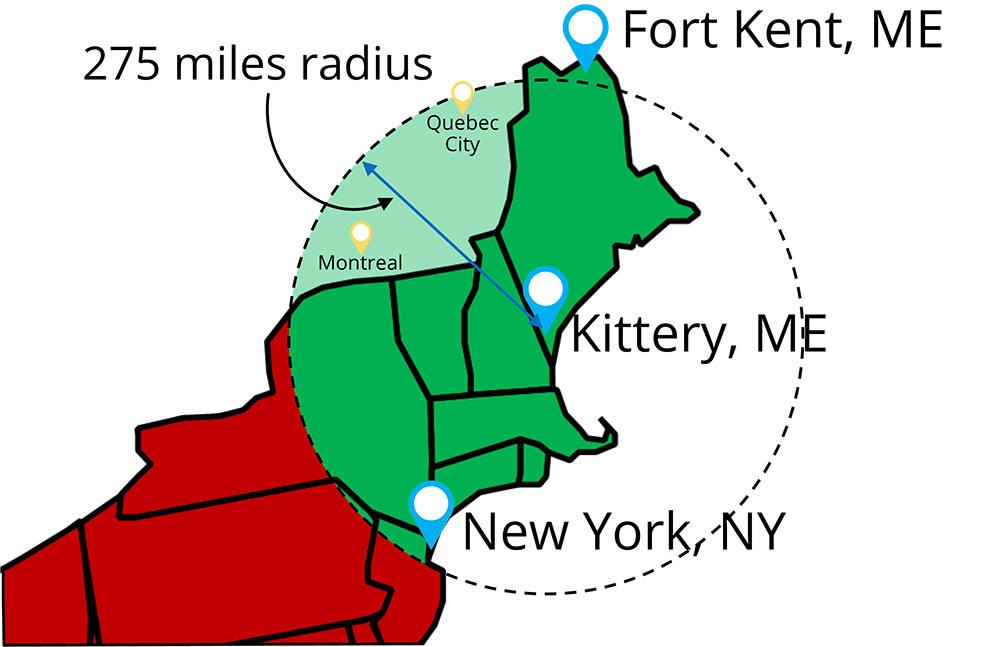
To help you understand the criteria for the qualified exemption, we created an illustration to help you understand what “qualified end-user” means. If your customers are buying for their own consumption, or are a restaurant or a retail food establishment, and they are in your state or within 275 miles “as the crow flies” from your facility, they are “qualified end-users.”
In the example illustrated, a company in Kittery, Maine, can sell to a customer from Presque Isle, Maine, because it is in-state (even if it is further away than 275 miles). The Kittery business can also sell to anyone in Vermont, New Hampshire, Massasuchetts, Connecticut, Rode Island, and parts of Pennsylvania, New Jersey, and New York, including New York City, which is inside the 275 miles limit. In the illustration, the business in Kittery can sell to anyone in the green zone.
To determine the radius for your business, use the radius map tool. You use this tool by entering the address of your facility as the “Input Point” using option two and click on “Draw Radius.” Add the radius area to the area of your state that is outside such radius, and you should have a map of your qualified end-users (assuming they are someone who is buying for their consumption, a restaurant, or a retail food establishment).
Now that you know the area for your qualified end-users, use the sales calculations to check if you are a qualified facility using our calculator tool. This tool was created to be as simple as possible. To use it, you will need to have the following information (the tool will calculate for inflation automatically):
A – Average annual sales to qualified end-users over the last three years.
B – Average annual sales to NON-qualified end-users over the last three years.
C – Average value of food manufactured, processed, packed, or held without a sale over the last three years.
After entering this data on the Microsoft Excel® spreadsheet tool, you will be answered about your eligibility to the qualified facility exemption. Download the calculator tool (Excel).
These new regulations may be intimidating for food entrepreneurs. However, this new approach to food safety is an important set of tools to identify and control risks to reduce foodborne illnesses. As the food economy becomes increasingly more globalized, these improvements are timely and should help provide safer food products in the U.S. and worldwide.
For more information on workshops and resources visit the Food Safety Training Opportunities website.
For more information on the Preventive Controls Rule or for upcoming courses, contact Robson Machado at 207.581.3144 or robson.machado@maine.edu.
References
- FDA — FSMA Final Rule for Preventive Controls for Human Food
- FDA — Key Facts about Preventive Controls for Human Food (PDF)
Information in this publication is provided purely for educational purposes. No responsibility is assumed for any problems associated with the use of products or services mentioned. No endorsement of products or companies is intended, nor is criticism of unnamed products or companies implied.
© 2018, 2020
Call 800.287.0274 (in Maine), or 207.581.3188, for information on publications and program offerings from University of Maine Cooperative Extension, or visit extension.umaine.edu.
The University of Maine System (the System) is an equal opportunity institution committed to fostering a nondiscriminatory environment and complying with all applicable nondiscrimination laws. Consistent with State and Federal law, the System does not discriminate on the basis of race, color, religion, sex, sexual orientation, transgender status, gender, gender identity or expression, ethnicity, national origin, citizenship status, familial status, ancestry, age, disability (physical or mental), genetic information, pregnancy, or veteran or military status in any aspect of its education, programs and activities, and employment. The System provides reasonable accommodations to qualified individuals with disabilities upon request. If you believe you have experienced discrimination or harassment, you are encouraged to contact the System Office of Equal Opportunity and Title IX Services at 5713 Chadbourne Hall, Room 412, Orono, ME 04469-5713, by calling 207.581.1226, or via TTY at 711 (Maine Relay System). For more information about Title IX or to file a complaint, please contact the UMS Title IX Coordinator at www.maine.edu/title-ix/.