Climate Highlights: Maine’s Climate Future: Ocean Acidification and Maine’s Seafood Supply
By Catherine Schmitt
The oceans cover seventy percent of Earth. The watery surface continually exchanges energy and chemicals with the overlying air in a constant pursuit of equilibrium. In this way, much of what humans put into the atmosphere eventually ends up in the ocean, including carbon dioxide.
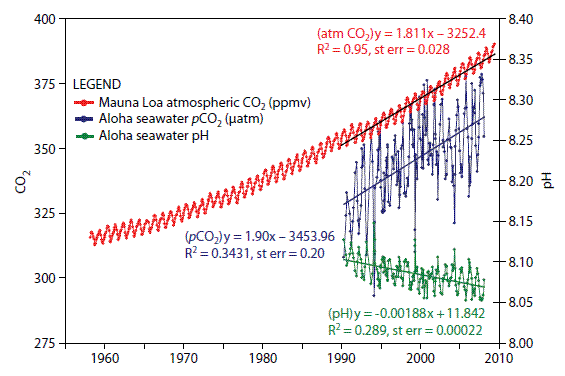
As carbon dioxide has increased in the atmosphere at an accelerated rate, so has the ocean absorbed carbon dioxide at an accelerated rate. This ocean “uptake” or “storage” accounts for approximately one-third of the carbon dioxide added to the atmosphere in modern times.
The best record of ocean acidity comes from the Mauna Loa observatory in Hawai’i. This record, combined with data inferred from samples of fossil shells, shows that the current rate of ocean acidification is ten times faster than at any time since the demise of the dinosaurs 65 million years ago.
What happens to carbon dioxide in the ocean?
In the ocean, carbon dioxide bonds with water to form carbonic acid, a weak, unstable acid that readily breaks apart into its components, bicarbonate, and hydrogen.
More carbon dioxide going into the ocean means more free hydrogen atoms (or ions), which are responsible for an increase in the ocean’s acidity, a process known as acidification.
It also means that more carbon is tied up as bicarbonate, leaving less carbonate available for the organisms that need it to build their shells and skeletons. In the Gulf of Maine, where so much of the marine resource economy (as much as 90%) depends on harvesting shelled animals, ocean acidification has the potential for devastating impacts.
What does ocean acidification mean for marine plants and animals?
The potential for ocean acidification to affect seafood has led the United Nations Environment Program to declare ocean acidification a threat to global food security.
Beneath the ocean surface, marine plants and animals also exchange minerals, nutrients, and other elements with the surrounding water. For example, clams, oysters, lobsters, urchins, sea stars, coral, and certain plankton use carbonate along with calcite and aragonite from seawater to build their shells and skeletons. Some fish have calcium carbonate in their scales. Under more acidic conditions, carbonate becomes scarce and shell-building can require more energy. Under very acidic conditions, the water (and seafloor sediment) actually becomes corrosive, and shells can begin to dissolve.
Monitoring for water and sediment acidity in the Gulf of Maine is just barely getting started. For now, most of what we know about the potential impacts from ocean acidification comes from recent laboratory experiments, and field studies in other parts of the world. The evidence from these studies suggests that ocean acidification threatens our valuable seafood supply. Shelled animals like lobsters, oysters, urchins, and clams may have trouble building shells, reproducing, and surviving. Acidic conditions may prove damaging to plankton, the tiny plants, and animals that form the base of the diverse and productive marine food web in the Gulf of Maine.
Increasing acidity is a threat to the entire ocean, but acidification isn’t a uniform phenomenon. Without studies specifically focused in Maine waters, it is difficult to know for sure how ocean acidification will impact our marine resources. The Gulf of Maine is a semi-enclosed sea with multiple sources of freshwater input, which may result in some areas being more or less acidic. However, many Gulf of Maine animals migrate here from other regions, such as the Arctic, where acidification effects are magnified. Other factors that influence the chemical balance of the Gulf of Maine include salinity, temperature, and distance from shore. Only with sustained monitoring will we have the information we need to anticipate how the chemistry of the Gulf of Maine and the valuable marine species that depend on it will change.
References and Resources
Doney, S.C., W.M. Balch, V.J. Fabry, and R.A. Feely. 2009. Ocean acidification: a critical emerging problem for ocean sciences. Oceanography 22:16-25.
Feely, R.A., S.C. Doney, and Cooley. 2009. Present conditions and future changes in a high-CO2 world. Oceanography 22:36-47.
National Research Council. 2011. Ocean acidification: a national strategy to meet the challenges of a changing ocean (2010). National Academies Press.
