222-Leaf and Soil Sampling Procedures
Fact Sheet No. 222, UMaine Extension Bulletin #2092
Originally by David E. Yarborough, Extension Wild Blueberry Specialist, and John M. Smagula, Emeritus Professor of Horticulture, The University of Maine, Orono, ME 04469. Revised July 2017.
Reviewed and updated by Lily Calderwood, Ph.D., University of Maine Cooperative Extension Wild Blueberry Specialist, 2023.
Leaf Sampling
Leaf sampling for nutrient levels is standard practice in the production of perennial crops. Leaf sampling will aid in determining fertilizer needs of your wild blueberry plants. Satisfactory leaf nutrient levels were established in Maine by Trevett in 1972 but have been updated by Santiago in 2011 (see the Wild Blueberry Fact Sheet No. 223, Interpreting Your Leaf Analysis Results, for details).
Regular leaf and soil sampling, done in the non-fruiting year, and visual monitoring of plants will help determine the overall health of your wild blueberry stand. A healthy stand has a high density of stems and bright, green leaves. Discolored leaves may indicate a nutrient deficiency. Wild Blueberry Fact Sheet No. 223, Interpreting Your Leaf Analysis Results, describes deficiency symptoms for nitrogen, phosphorus, and potassium.
The appearance of apparent mineral deficiency symptoms may be the result of an actual deficiency or could be caused by several other conditions or combinations of conditions including:
- Insufficient or poor soil moisture distribution, and/or topsoil erosion.
- Poor drainage and subsequent restriction of the root system.
- Insects, disease, fertilizer burn, weeds, or compaction of the soil, all of which weaken the root system.
- Periods of cool weather during the growing season.
- Injury from herbicides.
You should be sure that none of the above conditions exist before making additional fertilizer applications.
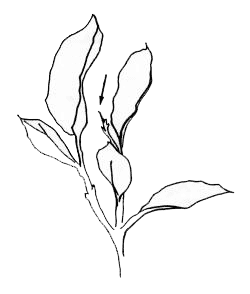
Leaf Sampling Procedure
Begin sampling when new stems, the summer after pruning, reach 90-100% tip-dieback (see Figure 1). Tip-dieback occurs when the newly emerging stems stop elongation: in Maine, this will generally occur around the 4th of July. Sampling should be limited to Vaccinium angustifolium (sweet low) and the sour top blueberry should not be sampled.
Fields should be divided into sampling areas. Low spots, trouble spots, and areas with obvious differences in soil type should be treated as separate sampling areas.
Stems should be taken from the area to be sampled by walking in a zig-zag fashion, cutting a total of about 90 stems. Cut stems at ground level since all the leaves will be included in the sample. If you strip all the leaves, the cost of the sample will be reduced. Do not include any soil particles on plants or crop year stems, and avoid sampling areas of severe disease or insect defoliation.
If the leaves are dusty, have soil on them, or you can see pesticide drift on the leaves, wash them. To wash, simply rinse the stems in water, shake as much water off as possible and lay them on paper towels until the surface is dry.
NOTE: You can receive a reduced price for the analysis if you strip the leaves from the stems and put just the leaves in the sample bag.
Samples should be stored in an open paper bag under dry conditions and mailed or taken to the Analytical Lab, Maine Soil Testing Services, 5722 Deering Hall, Orono, ME 04469-5722, as soon as possible to avoid losses in the leaf tissue nutrients. Cutting and bagging the stems the day of, or evening before they are sent to the University lab will ensure good quality samples. Pre-labeled tissue bags are available from the Wild Blueberry office at the University of Maine or at your local county Extension office. The County Extension offices and Blueberry Hill Farm in Jonesboro, ME will also accept leaf samples to be taken to the University of Maine Analytical Laboratory.
Soil Sampling Procedure
Soil sampling helps to determine soil pH. Ideal soil pH is about 4.0. When the pH is within this range, the nutrients needed for wild blueberry growth are still available to wild blueberry plants but weed completion is reduced. When pH is above 4.0 you need to add sulfur to bring the pH down to 4.0. Standard soil nutrient levels tests for nutrients may not be used to determine optimum wild blueberry production. Soil samples can be taken at any time. It may be most convenient to take them when leaf sampling is at the tip-dieback stage.
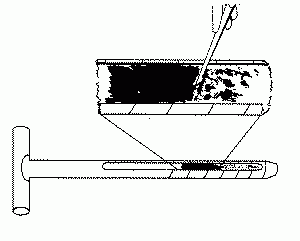
Instructions for sampling soil appear on page 4 of the University of Maine Analytical Lab’s Maine Soil Testing Service, The University of Maine, Field and Soil Sample Information Form (PDF). These instructions should be followed except only the top 3 to 4 inches, including the surface organic (pad) layer or the dark brown upper layer, should be included in your sample (see Figure 2).
Place samples in a sturdy container, such as a pint ice cream carton or a University soil sample box available from the Analytical Lab or at your local county Extension office and at Blueberry Hill Farm. Indicate that you want pH only, there is no need to fill out the soil test form.
Mail to:
Analytical Lab and Maine Soil Testing Service
5722 Deering Hall
Orono, ME 04469-5722
You may also give it to your local county Extension office or Blueberry Hill Farm to be transported to the University of Maine.
Test Results
The University of Maine Analytical Lab will analyze the samples for available nutrients. The results will be sent directly to you with the appropriate fertilizer or sulfur recommendations for your field (see Figure 3).
In instances where leaf nutrient levels are below the satisfactory level, an application of a particular nutrient may be suggested. Samples with nutrient levels near the recommended level may indicate you need to try further evaluation in your field with test strips.
Whenever fertilizer is applied to a field, it is recommended that three, unfertilized field strips be staked out, at least 100′ long and 10′ wide. These strips will help determine the effect of the fertilizer application when compared to strips of equal size in the adjacent fertilized area. Mark the fertilized strips with semi-permanent markers (A 6-inch long, 1-inch diameter PVC pipe driven almost to ground level works well).
To verify that the fertilizer application has raised the levels to the optimum level you should also take leaf samples from the field the year you apply the fertilizer.
Figure 3: Example of a Foliar Analysis Report
| University of Maine Analytical Lab Lowbush Blueberry Foliar Analysis Report |
||||
| Washington County – ICM Program P.O. Box 121 Machias, Maine 04654 |
Job #1684 | |||
| Lab # 195 | ||||
| Field Name: Example of Maine Field | ||||
| __________________________________________ | ||||
| Nutrient | Level Found | Foliar Levels | ||
| Santiago 2011 | Optimum | |||
| Nitrogen | (%) | 2.01 | 1.55-1.85 | 1.76 |
| Calcium | (%) | 0.477 | 0.31-0.40 | 0.38 |
| Potassium | (%) | 0.564 | 0.31-0.56 | 0.44 |
| Magnesium | 0.217 | 0.16-0.18 | 0.17 | |
| Phosphorus | 0.149 | 0.111-1.43 | 0.136 | |
| Aluminum | (ppm) | 84.9 | 98-289 | 179 |
| Boron | (ppm) | 23.6 | 2-44 | 23 |
| Copper | (ppm) | 5.50 | 3-6 | 4 |
| Iron | (ppm) | 37.1 | 50-100 | 35 |
| Manganese | (ppm) | 1010 | 710-2637 | 963 |
| Zinc | (ppm) | 20.3 | 10-15 | 13 |
| Soil pH | 5.1 | 4.0 | ||
| Recommended Nutrient Amendments Foliar levels of both N and P are optimum. No yield response is expected from fertilizer application. Soil pH should be lowered to target level of 4.0. Apply 900 lb./a yellow (elemental) sulfur per acre. To verify that the fertilizer application has raised the levels to the optimum level you should also take leaf samples from the field the year you apply the fertilizer. |
||||
Information in this publication is provided purely for educational purposes. No responsibility is assumed for any problems associated with the use of products or services mentioned. No endorsement of products or companies is intended, nor is criticism of unnamed products or companies implied.
© 2017, 2023
Call 800.287.0274 (in Maine), or 207.581.3188, for information on publications and program offerings from University of Maine Cooperative Extension, or visit extension.umaine.edu.
In complying with the letter and spirit of applicable laws and pursuing its own goals of diversity, the University of Maine System does not discriminate on the grounds of race, color, religion, sex, sexual orientation, transgender status, gender, gender identity or expression, ethnicity, national origin, citizenship status, familial status, ancestry, age, disability physical or mental, genetic information, or veterans or military status in employment, education, and all other programs and activities. The University provides reasonable accommodations to qualified individuals with disabilities upon request. The following person has been designated to handle inquiries regarding non-discrimination policies: Director of Equal Opportunity, 5713 Chadbourne Hall, Room 412, University of Maine, Orono, ME 04469-5713, 207.581.1226, TTY 711 (Maine Relay System).
