Soil, Air & Water Exercises
Soil, Air & Water Exercises (PDF)
-
- How Soil is Formed: You can demonstrate some of the physical forces of nature that break up rocks to form soils.
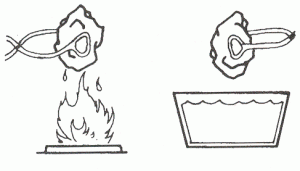
- Heat and Cold (ask your leader to help)
- Using tongs, hold a piece of limestone over a flame or stove, being very careful not to burn yourself!
- Drop the hot rock into a pan of cold water. Record what happens. Explain how the heat of summer and cold winter can break rocks into smaller pieces.
- Freezing and Thawing
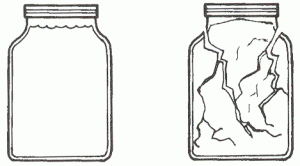
- Fill a small jar completely with water. Screw the lid on tightly.
- Place the bag in a freezer and leave it long enough to completely freeze the water in the jar.
- Carefully remove the towel and jar from the bag. Record the effect of freezing on the glass. What happened? Why? How does that relate to soil formation?
- Wind and Water
- Rub two soft stones or rocks together.
- Record what happens. How does this demonstrate the effects of wind and water?
- Water: Sodium chloride is a mineral compound that makes up table salt.
- Put one tablespoon of table salt in a glass of water and stir.
- Record what happens. How does this relate to soil formation?
- Heat and Cold (ask your leader to help)
- How Soil is Formed: You can demonstrate some of the physical forces of nature that break up rocks to form soils.
All of these factors — heat, cold, freezing, thawing, wind and water — are vital to the development of new soil. The term “weathering” is used to include the effects of all of these factors. In your own words, explain the term “weathering” and how it relates to soil formation.
- Separating Sand, Silt and Clay: Sand, silt and clay are the primary components of soil. To see them, try this exercise.
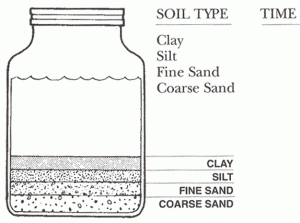
- Get a quart of soil from the garden, flower bed or field. Empty onto newspaper, and let it dry.
- Crush any lumps.
- Remove trash, rocks and roots.
- Fill a quart jar 1/4 full with dry soil.
- Add water until the jar is 3/4 full.
- Add a tablespoon of non-foamy detergent.
- Close the lid tightly and shake hard for about three minutes. Keep shaking until all the particles are separated from each other.
- Set the jar on a table and watch very closely for 10-15 minutes. Write down what you see happening. Do not disturb the jar for two days.
- Place an index card along side the jar. Mark off the depth of each layer that you can see in the jar. These layers are the clay, silt, find sand and coarse sand. Label your card for each layer illustrated.
- Shake the jar again to mix up the layer. Set the jar down hold your card beside it. Note how long it takes for each layer to reform. You may have to leave the jar for a while before you can see the upper layers form. Explain this difference in time.
- Discuss why each layer of soil is necessary for growing plants. You may have to do some reading to find the answers!
- Air Has Weight
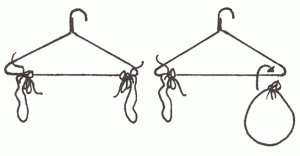
- Take two empty balloons of equal size and weight and attach them with two equal lengths of string to two corners of a wire coat hanger. Balance it as shown in the diagram.
- Remove one balloon, blow it up, and attach it again to the coat hanger.
- Record what happens. How does this show that air has weight?
- Air Has Volume
- Attach two inflated balloons of the same size to the two ends of a straightened coat hanger and balance them so that the hanger is level.
- Hold one of the balloons under hot water for a few minutes.
- Record what happens to the balloon.
- Air Contains Solid Particles
- Coat a glass slide, a petri dish (see your science teacher), or a piece of white paper with a very thin layer of petroleum jelly.
- Place it outside, on a window sill or table, on a day when there is no wind.
- After an hour or two, bring it inside and examine it. What has collected on the sticky surface? Is it dirty or dark? Can you guess where these particles have come from?
- Repeat the same experiment on a windy day.
- Do you get more particles on your sticky surface on a calm day or a windy day? Why?
- Water Molecules Bond Together
- Fill a narrow-mouth glass, such as a bud vase, half full with water.
- Observe the edge of the water surface where it touches the side of the glass. Record your observations.
- Fill the glass almost to the top. Using an eye dropper, continue adding water one drop at a time until it overflows. Record your observations. What did you notice about the level of the water at the center of the vase just before it overflowed? What about at the edge of the rim just before it overflowed? This experiment shows you strength of the bonds between water molecules that result in water’s surface tension.
- How Much Water Is In You?
- Use a scale to determine your body weight.
- The human body is about 80 percent water. How many pounds of your body weight is water?
- Melting Rates of Ice
- Make some ice cubes that have the same amount of water in them.
- Give each person an ice cube to hold. You can use your hand or a cup, but everyone has to use the same thing.
- Record how long it takes for each person’s ice cube to melt. Why do the cubes melt at different rates?
- How Fast Does Water Freeze?
- Boil some water and pour it into a heat-proof cup. Steam is very hot, so be careful not burn yourself!
- Put the same amount of room-temperature tap water in another cup.
- Put the same amount of cold tap water in another cup.
- Place all the cups in the freezer. Check them after an hour. Which freezes faster? Why?
- Make It Rain!
- Boil some water in a kettle.
- Turn the heat off. Wearing oven mitts, and being very careful not to get burned by the hot steam, hold a large glass or bowl upside down over the rising steam. What happens? Why? How is this like rain forming and falling?
- Temperature and Rainfall
- Keep a daily record of the weather. Include the high and low temperature, the amount of rain (or snow) and the temperature when it was raining ( or snowing).
- What was the total amount of rain (or snow) each month?
- Can you find any relationship between temperature and rainfall (or snowfall)? What is that relationship? Why?
- Acid Precipitation: Rain, snow, sleet or fog that contains certain pollutants is called acid precipitation.
- Collect samples of rain at the beginning, in the middle and at the end of a storm.
- Measure the acidity of the water using paper that is sensitive to pH. Your science teacher or Extension educator can help you find out where to get this.
- Record your observations. During what part of the rain storm is the water most acidic (lowest pH number)? Why?
- What do you think causes acid precipitation? Do some research into sources of pollutants that cause acid precipitation, and the effects of acid precipitation on plants and animals.
